Title: Roflumilast
CAS Registry Number: 162401-32-3
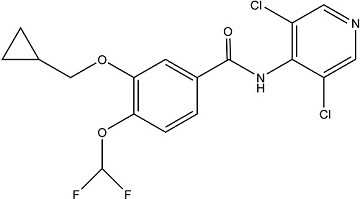
CAS Name: 3-(Cyclopropylmethoxy)-N-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)benzamide
Manufacturers' Codes: BY-217
Trademarks: Daxas (Altana)
Molecular Formula: C17H14Cl2F2N2O3
Molecular Weight: 403.21
Percent Composition: C 50.64%, H 3.50%, Cl 17.59%, F 9.42%, N 6.95%, O 11.90%
Literature References: Selective phosphodiesterase 4 (PDE4) inhibitor. Prepn: D. Flockerzi et al., WO 9501338; H. Amschler, US 5712298 (1995, 1998 both to Byk-Gulden). Pharmacology: A. Hatzelmann, C. Schudt, J. Pharmacol. Exp. Ther. 297, 267 (2001); D. S. Bundschuh et al., ibid. 280. Clinical evaluation in allergic rhinitis: B. M. W. Schmidt et al., J. Allergy Clin. Immunol. 108, 530 (2001); in exercise-induced asthma: W. Timmer et al., J. Clin. Pharmacol. 42, 297 (2002); in COPD: K. F. Rabe et al., Lancet 366, 563 (2005). Comparative review with cilomilast: M. A. Giembycz, Monaldi Arch. Chest Dis. 57, 48-64 (2002).
Properties: Crystals from isopropanol, mp 158°.
Melting point: mp 158°
Therap-Cat: Antiasthmatic; in treatment of chronic obstructive pulmonary disease.
Keywords: Antiasthmatic (Nonbronchodilator); Phosphodiesterase Inhibitor.
Tianjin Chase Sun Pharmaceutical Co. Ltd was founded in 1996 and the joint-stock transformation was completed in 2000. In 2009, Chase Sun pharmaceutical was listed on the Shenzhen Stock Exchange and became the first group of national GEM listed companies, also called as the first of Tianjin(Stock code: 300026). In 2013, the company won top 20 of the most competitive pharmaceutical listed Company in China.
Products: Xuebijing Injection (Modern Traditonal Chinese Medicine), Fasudil hydrochloride oral preparation; And anti-tumor, immunoregulation, Cardiovascular and cerebrovascular, Anaesthesia API and intermediates;
Technology Transfer Center
Technology transfer center of Chase Sun Pharma covers an area of 25, 000 square meters with a total investment of 300 million Yuan. Technology transfer center possesses production lines for synthetic drugs, such a polusaccharide, peptides, anti-cancer drugs and production lines for large volume injection, small volume injection, freeze-dried powder and oral solid preparation. Each line is constructed in accordance with the GMP Standard (2010 revision) and has a laboratory for comprehensive testing and analysis with a complete production quality control system. Technology transfer center is committed to providing the customers services of process development of APIs and the intermediates from pre-clinical to the industrialization.
Services (Product means API, intermediate and fine chemical)
1: (GMP & Non GMP); Own product production, sales;
2: (GMP & Non GMP); Product Contract Manufacturing;
3: \Product research and development, achievements transformation including pilot studies and scale-up trials for new preparation and new products;
4:; Studies of controlling the conditions and process of production;
5:; Validation of the maturity of the technical;
6:; Providing drugs for clinical trials and small batch production;
7: \; Technology research, pilot project and technology programs advising and services;
8:; Drug registration advising and services;